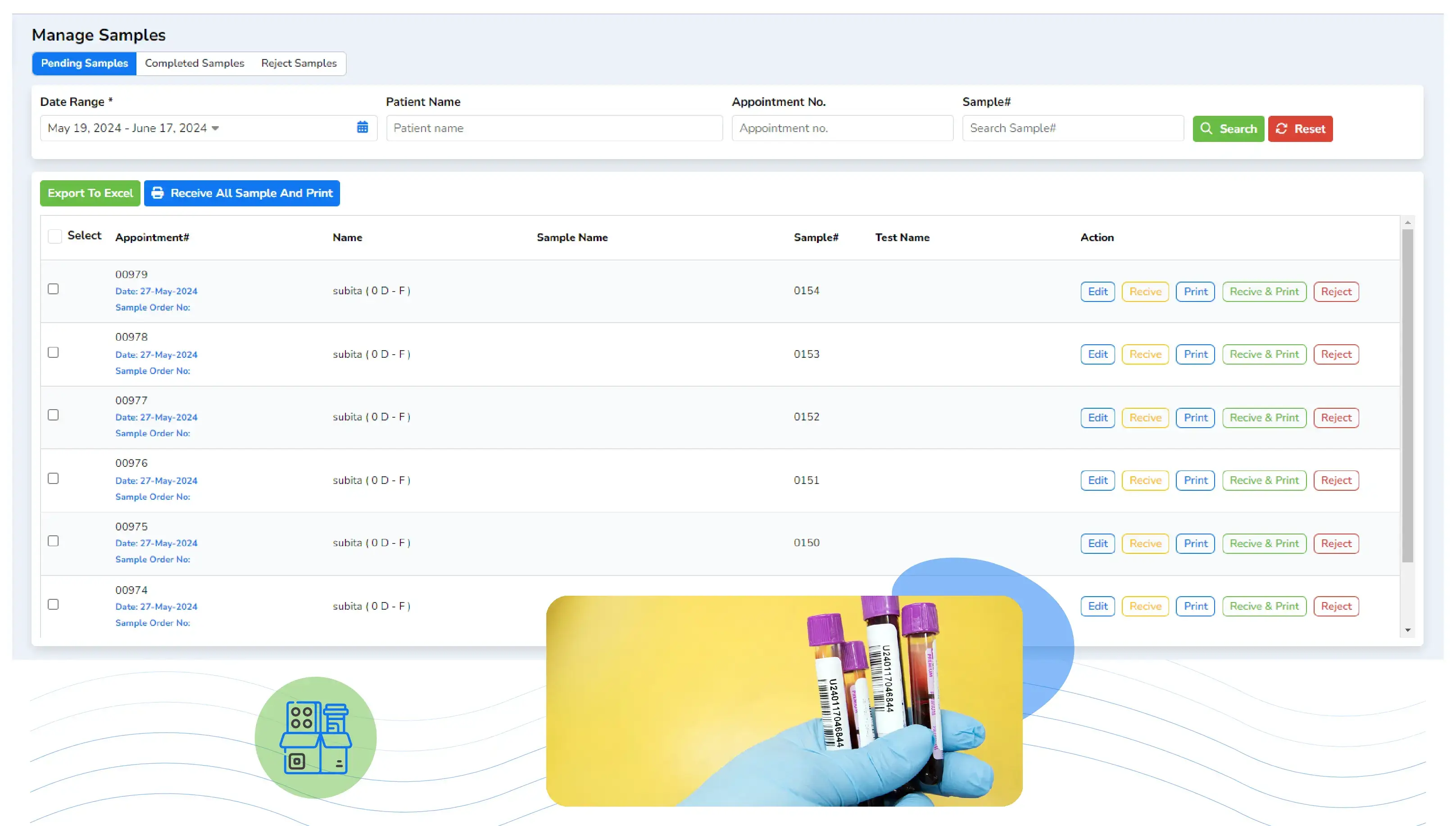
Laboratory Information Management Systems (LIMS) have become a cornerstone in the management and operation of medical laboratories, particularly in specialized fields like pathology and radiology. These systems streamline workflows, enhance data management, and ensure compliance with industry regulations. For labs seeking accreditation, LIMS plays a crucial role in meeting the standards set by regulatory bodies, ensuring high-quality services, and improving patient outcomes.
In this article, we will explore how LIMS supports pathology and radiology labs in achieving accreditation, focusing on its benefits, functionality, and the role it plays in meeting the rigorous requirements set forth by accrediting organizations.
1. Understanding Accreditation in Pathology and Radiology Labs
Before delving into the specifics of how LIMS aids in accreditation, it’s important to understand the significance of accreditation in healthcare laboratories. Accreditation ensures that a laboratory meets the highest standards of quality and safety in patient care, diagnostics, and testing.
Key Accreditation Bodies in Pathology and Radiology:
- College of American Pathologists (CAP): Accredits pathology laboratories to ensure they meet the highest standards in diagnostic accuracy, safety, and patient care.
- The Joint Commission (TJC): Accredits a wide range of healthcare facilities, including radiology labs, ensuring that they adhere to comprehensive standards for quality and safety.
- International Organization for Standardization (ISO): ISO certifications like ISO 15189 are crucial for both pathology and radiology labs, ensuring they meet international quality management standards.
- American College of Radiology (ACR): Accredits radiology labs to ensure that they meet essential standards for diagnostic imaging, reporting, and patient care.
Achieving accreditation from these bodies requires labs to adhere to strict protocols, maintain consistent quality, and demonstrate compliance with industry regulations. LIMS acts as a powerful tool in meeting these requirements.
2. LIMS and Standardization of Processes
One of the primary benefits of LIMS is its ability to standardize laboratory processes, which is essential for accreditation. Accrediting bodies require labs to follow specific protocols for test procedures, sample handling, reporting, and data management. LIMS helps ensure that these processes are consistently followed, minimizing human error and increasing the reliability of test results.
How LIMS Contributes to Standardization:
- Standard Operating Procedures (SOPs): LIMS allows for the automation and enforcement of SOPs across all processes, ensuring that laboratory staff follows the same procedures for sample collection, analysis, and reporting.
- Tracking and Documentation: LIMS provides a comprehensive record of each step in the laboratory workflow, allowing for easy tracking and documentation of compliance with SOPs. This is crucial for audits and inspections during the accreditation process.
- Error Reduction: By automating tasks such as data entry, sample tracking, and result generation, LIMS reduces the chances of human error, ensuring that laboratories consistently meet the high standards required for accreditation.
3. Ensuring Data Integrity and Traceability
Accreditation standards require laboratories to maintain accurate, reliable, and traceable records of all patient data and test results. Data integrity is one of the most critical aspects of achieving and maintaining accreditation. LIMS ensures that data is captured, stored, and managed securely, preventing errors or unauthorized access that could compromise the quality of diagnostic services.
Key Features of LIMS for Data Integrity:
- Audit Trails: LIMS automatically generates audit trails, which record every action taken on patient data, including who accessed the data, when it was accessed, and what changes were made. This feature ensures that all actions are traceable, which is essential for meeting accreditation requirements.
- Data Validation: LIMS systems can be configured to validate data inputs at every stage, ensuring that test results, sample information, and patient details are accurate and free from errors. This feature enhances the quality and reliability of results.
- Access Control: LIMS provides secure access controls, ensuring that only authorized personnel can enter, modify, or view sensitive patient information. This is particularly important for maintaining compliance with regulations like HIPAA (Health Insurance Portability and Accountability Act) in the U.S.
By ensuring data integrity and traceability, LIMS helps labs meet the documentation and record-keeping requirements set by accreditation bodies.
4. Regulatory Compliance and Quality Management
Achieving accreditation requires that laboratories comply with various regulatory standards that govern their operations. LIMS is specifically designed to help labs meet these standards by automating compliance tasks and offering tools for quality management.
LIMS and Compliance:
- Regulation Adherence: LIMS is capable of integrating with various national and international standards, ensuring that lab operations align with the regulations of accrediting bodies. Whether it’s ensuring that test results are delivered within specific time frames or that sample processing is done in accordance with health and safety regulations, LIMS helps maintain compliance.
- Quality Control (QC) and Quality Assurance (QA): LIMS offers powerful QC/QA features, allowing labs to track the quality of their testing processes and detect any deviations from established standards. This feature is vital for maintaining consistent quality and achieving accreditation.
- Inspection Readiness: LIMS stores all relevant data, reports, and documents in a centralized, organized system, making it easy for labs to prepare for inspections by accrediting bodies. During audits, LIMS provides auditors with easy access to the necessary records, streamlining the accreditation process.
By supporting regulatory compliance and ensuring robust quality management, LIMS helps labs adhere to the stringent standards set by accrediting organizations.
5. Enhancing Laboratory Workflow and Efficiency
In addition to its role in compliance and data management, LIMS also improves laboratory workflow and efficiency, which is essential for meeting accreditation standards. Accrediting bodies often assess how well a lab manages its resources, processes, and personnel to ensure optimal efficiency and patient care.
How LIMS Improves Workflow Efficiency:
- Automation of Routine Tasks: LIMS automates many routine tasks, such as sample tracking, data entry, and report generation. This automation frees up laboratory staff to focus on more complex tasks, improving overall productivity.
- Real-Time Monitoring: LIMS provides real-time monitoring of the laboratory workflow, enabling staff to identify bottlenecks, delays, or inefficiencies that may hinder the lab’s ability to meet accreditation timelines.
- Resource Management: LIMS can help labs optimize resource utilization, including personnel, equipment, and materials. By tracking inventory levels and scheduling maintenance for equipment, LIMS ensures that labs have the necessary resources to operate smoothly and maintain accreditation standards.
By improving workflow efficiency, LIMS helps labs meet the operational benchmarks set by accreditation bodies while enhancing overall service quality.
6. Documentation and Reporting for Accreditation
Accreditation bodies require comprehensive documentation that demonstrates a lab’s adherence to established standards. LIMS plays a vital role in documentation and reporting, allowing labs to easily generate reports that prove their compliance with regulatory requirements.
LIMS and Documentation:
- Customizable Reports: LIMS allows labs to generate customizable reports for regulatory bodies, including test results, quality control data, audit trails, and other compliance-related information. These reports can be tailored to meet the specific needs of accreditation organizations.
- Real-Time Data Access: LIMS provides real-time access to critical data, making it easier for labs to quickly provide documentation during audits or inspections.
- Compliance Checklists: LIMS can also generate automated compliance checklists, helping labs ensure that all accreditation requirements are being met before submitting their application.
By simplifying the documentation and reporting process, LIMS makes it easier for labs to maintain their accreditation status.
Conclusion
In conclusion, LIMS plays an essential role in helping pathology and radiology labs achieve and maintain accreditation. By standardizing processes, ensuring data integrity, supporting regulatory compliance, and improving workflow efficiency, LIMS enables labs to meet the high standards set by accreditation bodies. With its robust features and automation capabilities, LIMS not only helps labs streamline operations but also enhances diagnostic accuracy, reduces human error, and ultimately leads to better patient care. As healthcare regulations continue to evolve, LIMS will remain a key tool for laboratories striving for excellence and accreditation.
About the Author: Morain is an experienced content writer and SEO expert based in Mumbai, India. With 10 years of expertise in the healthcare SaaS industry, he specializes in creating optimized content that drives results and helps businesses succeed online. His deep understanding of SEO strategies and healthcare technology allows him to craft content that not only informs but also ranks well on search engines.